Salivary Glycoproteins
Saliva is a readily accessible secretion that plays an important role in the maintenance of oral homeostasis, tooth integrity, the initiation of digestion, and antimicrobial defense, which is secreted by three pairs of major salivary glands (parotid, submandibular and sublingual)and by many minor salivary glands. Saliva is mainly composed of water (-94%);however, the minor components include bioactive molecules that have crucial roles in many tasks. Human whole saliva has a protein content of approximately 0.5-3 mg/mL, and the large and diverse set of proteins perform multiple functions.
It has been reported that a significant amount of these proteins is modified by carbohydrates, and some proteins may contain up to 80% carbohydrate (e.g., MUC5B), but 10-40% carbohydrate is more typical of glycoproteins. The most important constituents include amino sugars, galactose, mannose, and sialic acid (N-acetylneuraminic acid), and the total amount of protein-bound carbohydrates in the saliva is 300-400 μ g/mL. These protein-bound carbohydrates have increased the viscoelasticity of the saliva, prevent proteolysis by keeping proteases at a distance, prevent acid precipitation in the case of several glycoproteins (e.g., acid-soluble blood group antigens, mucins), and serve labeling/antigen functions.
Protein glycosylation is the enzymatic addition of sugars or oligosaccharides to proteins. Two types of protein glycosylation exist: N-glycosylation to the amide nitrogen of asparagines (Asn) side chains and O-glycosylation to the hydroxyl groups of serine (Ser) and threonine (Thr) side chains. Glycosylation increases the diversity of the proteome to a level unmatched by any other post-translational modification due to the various aspects of lycosylation that can be modified, including glycosidic bond, lycan composition, glycan structure, and glycan length.
Approximately half of all proteins typically expressed in a cell undergo this modification.In the past few years, scientific interest n saliva has increased not only for the well-documented interactions with various diseases,but also for the significant age-or sex- related differences in genomic and proteomic expression in human parotid gland and saliva. Six proteins (e.g.,β-2-microglobulin and transferrin) have been identified with significant higher expression in females, while eight proteins (e.g.,prolactin inducible protein) in males and seven proteins (e.g.,Ig-k light chain and transferrin) in females showed significant age differences. However, little is known about the age- and sex-associated changes in the glycopatterns of human salivary glycoproteins, which, possibly, may provide more sensitive indicators for better diagnosis and treatment of many diseases and natural age- and gender-dependent expression of marker proteins. Recent researches demonstrated that salivary MUC5B consists of glycoforms with distinct glycosylation that vary extensively between individuals and that some of this variation is due to blood group and secretor status. Meanwhile, other studies reported an increase in total protein, secretory IgA,MUC5B, glycoprotein 340, and sialic acid, as well as a reduction in MUC7 with age in parotid saliva. It is known that many major diff erences in the physiology of men and women result from the activity of gonadal steroid hormones. Animal research and human studies have revealed sex differences with respect to both the composition and flow rate of saliva.
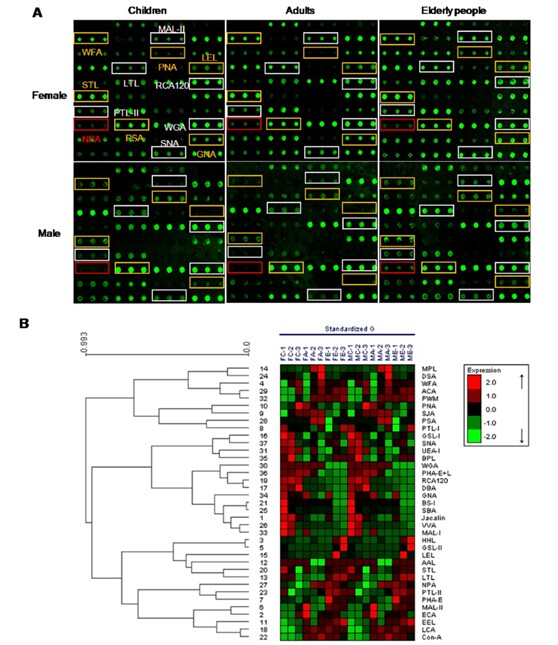
Glycopatterns of salivary glycoproteins from female and male children, adults and elderly groups using a lectin microarray, respectively.
(A) The profile of Cy3-labeled salivary proteins from female and male children, adults and elderly groups bound to the lectin microarrays, respectively. Fluorescent images were scanned at 70% photomultiplier tube and 100% laser power settings with a Genepix 4000B confocal scanner. A portion of the slide with three replicates of the lectin array was shown. The lectin microarrays revealed age difference marked with white frames and sex difference marked with yellow frames. (B) Heat map and hierarchical clustering analysis of the 37 lectins with three biological replicates. Samples were listed in columns and the lectins were listed in rows. The color and intensity of each square indicated expression levels relative to other data in the row. Red, high; green, low; black, medium. FC, female children; FA, female adults; FE, female elderly subjects; MC, male children; MA, male adults; and ME, male elderly subjects.
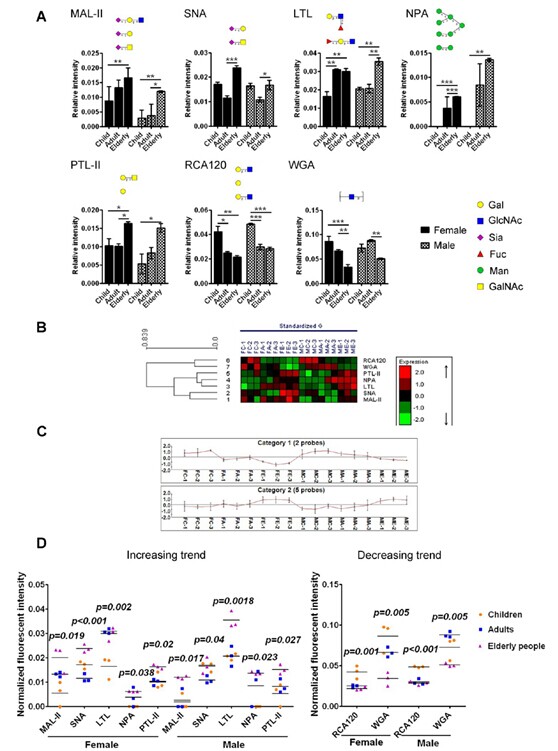
Relative expression levels of glycans that differed significantly with respect to age.
(A) Significant differences among children, adults, and elderly individuals in females and males were analyzed according to t-test, respectively ( * P < 0.05, ** P < 0.01, and *** P ≤ 0.001). The data were the averaged NFI ± SD of three biological replicates. (B) Hierarchical clustering analysis (HCA) of the lectin microarrays in six groups of saliva samples using seven lectins. (C) Lectins were categorized into two independent groups according to HCA, including decreasing trend and increasing trend of signals for lectins with age, respectively. (D) Scatter plot analysis of the lectin microarrays in six groups of saliva samples using seven lectins.
